中國杭州和紹興,2022年12月21日—歌禮制藥有限公司(香港聯交所代碼:1672,“歌禮”)的全資子公司甘萊製藥有限公司自主研發的甲狀腺激素受體β(THRβ)激動劑ASC41用於治療肝穿活檢證實的非酒精性脂肪性肝炎(NASH)患者的52周II期臨牀試驗入組順利推進。ASC41 II期臨牀試驗是由中國生物技術公司發起的目前進展最快的療程為52周入組肝穿活檢證實NASH患者的II期臨牀試驗,於2022年10月初完成首例患者入組。全部患者入組預計於2023年第三季度底完成。
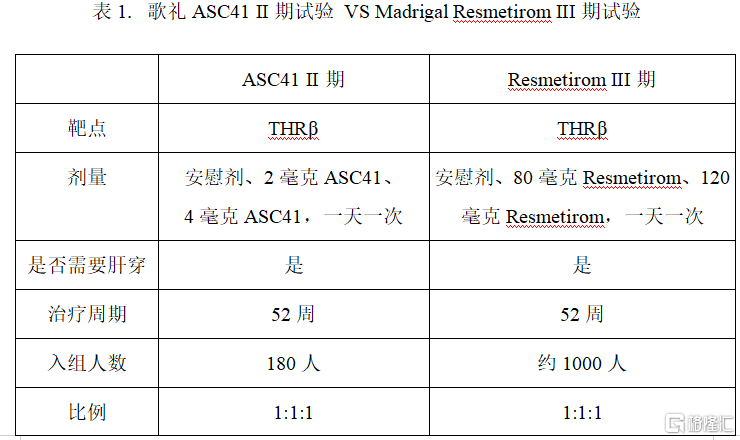
2022年12月19日,Madrigal Pharmaceuticals, Inc.公佈了Resmetirom (MGL-3196) 療程為52周的治療肝穿活檢證實的NASH患者III期臨牀數據。ASC41與Resmetirom臨牀試驗方案對比見表1。

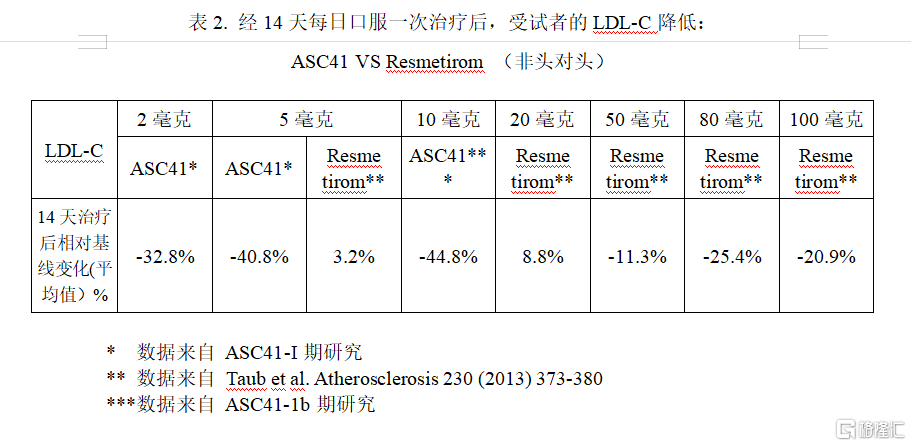
ASC41是歌禮自主研發的一款肝臟靶向性前體藥物,其活性代謝產物可選擇性激活甲狀腺激素受體β(THRβ)。2021年1月,歌禮宣佈ASC41完成一項隨機、雙盲、安慰劑對照、單劑量和多劑量遞增的I期臨牀試驗,試驗人羣為65位低密度脂蛋白膽固醇(LDL-C)大於110 mg/dL的受試者。經過14天每日口服一次ASC41片劑治療後,受試者的LDL-C顯著降低(https://www.ascletis.com/news_detail/175/id/454.html)。2021年2月,歌禮宣佈ASC41在超重和肥胖受試者中取得的良好的臨牀試驗結果。數據表明經過14天每日口服一次ASC41片劑治療後,受試者的LDL-C顯著降低.(https://www.ascletis.com/news_detail/175/id/462.html)。不同劑量的ASC41和Resmetirom每日口服一次治療後,LDL-C降低數據比較見表2。
2021年9月,歌禮在美國完成了ASC41口服片劑的健康受試者藥物相互作用和非酒精性脂肪性肝病(NAFLD)患者藥代動力學(PK)的I期臨牀試驗。ASC41主要通過CYP3A4代謝形成活性代謝物ASC41-A,ASC41-A是一種選擇性甲狀腺激素受體β激動劑。該臨牀研究結果表明ASC41/ASC41-A與NASH患者人羣常用的抗抑鬱藥物(選擇性5-羥色胺再攝取抑制劑(SSRIs)和5-羥色胺-去甲腎上腺素再攝取抑制劑(SNRIs),其中大部分為輕度/中度CYP3A4抑制劑)之間產生具有臨牀意義的藥物相互作用的可能性小;ASC41/ASC41-A的藥代動力學特徵在健康受試者與NAFLD患者中無顯著差異(https://www.ascletis.com/news_detail/175/id/537.html)。
關於歌禮
歌禮是一家在香港證券交易所上市(1672.HK)的創新研發驅動型生物科技公司,涵蓋了從新藥研發至生產和商業化的完整價值鏈。歌禮的管理團隊具備深厚的專業知識及優秀的過往成就,在團隊的帶領下,歌禮聚焦三大臨牀需求尚未滿足的醫療領域:病毒性疾病、非酒精性脂肪肝和腫瘤,並以全球化的視野進行佈局。憑藉卓越的執行力,歌禮快速推進藥物管線開發,爭取在國際競爭中佔據領先地位。歌禮目前擁有三個商業化產品,即利托那韋片、戈諾衞®和新力萊®,以及22款在研藥物。最前沿的候選藥物包括ASC22(乙肝功能性治癒)、ASC10和ASC11(口服小分子抗新冠藥)、ASC40(複發性膠質母細胞瘤)、ASC42(原發性膽汁性膽管炎)和ASC40(痤瘡)。
欲瞭解更多信息,敬請登錄網站:www.ascletis.com。
詳情垂詢:
歌禮制藥有限公司
+86-181-0650-9129
pr@ascletis.com
ir@ascletis.com


