機構:光大證券
評級:買入
◆事件:(1)公司公告,與軍事科學院軍事醫學研究院生物工程研究所聯合開發的全國首款重組新型冠狀病毒疫苗(腺病毒載體)(「Ad5-nCoV」)於2020年3月17日獲批進入臨牀試驗。(2)公司公佈2019年報:實現其他收益1900萬元;研發支出1.52億元,同比增長33.5%;税後綜合虧損總額1.56億元;每股收益-0.77元。
◆點評:
康希諾生物是國內高起點的疫苗研發型企業。公司擁有比肩國際質量標準的生產體系,研發具有全球視野。研發管線包含13個疾病領域16種疫苗產品。國內首款重組新冠疫苗獲批臨牀,是繼埃博拉疫苗之後研發實力的再次證明。此外肺炎、結核病、腦膜炎、百白破等疫苗品種研發進展順利。
腦膜炎疫苗:MCV4,已於2019年12月報產並納入優先審評。MCV2於2019年1月報產,目前已完成廠房及工藝驗證,預計2020年獲批。
肺炎疫苗:PCV13i,2019年4月獲批進入臨牀,採用CRM197、TT載體,有望成為中國最佳的13價肺炎結合疫苗。PBPV,是全球首個廣譜性肺炎球菌蛋白疫苗,已開始進行Ia期臨牀,並預計2020年完成Ia期臨牀試驗。
百白破疫苗:嬰幼兒用DTcP(組分百白破),已進入臨牀一期,預計於2022年完成臨牀三期。加強型DTcP,目前處於臨牀一期,預計於2021年完成臨牀三期。青少年及成人用Tdcp:計劃率先於海外開展臨牀,並於2020年底前在中國提交臨牀試驗申請。
結核病加強疫苗:全球首個成人結核病創新疫苗,針對4至18歲年齡組。已在Ia期臨牀中顯示出良好的安全性和免疫原性,正在加拿大開展臨牀Ib期,有計劃於2020年在國內直接進入臨牀二期。
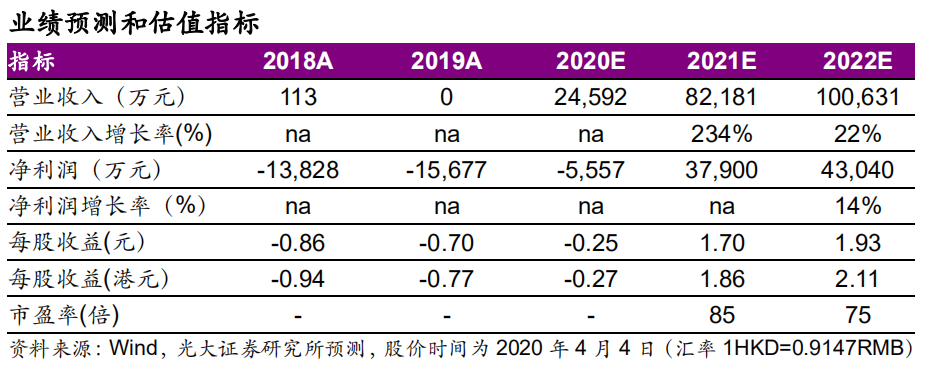
◆盈利預測與估值:因20年研發與銷售投入預計增長較快,略下調20-21年EPS至-0.25/1.70元(原0.31/0.88元),新增22年EPS1.93元。公司首款產品MCV2疫苗有望於2020年上市,重磅產品MCV4有望於2021年起貢獻業績,研發管線價值高,維持“買入”評級。
◆風險提示:新產品研發不達預期;行業競爭加劇的風險。